
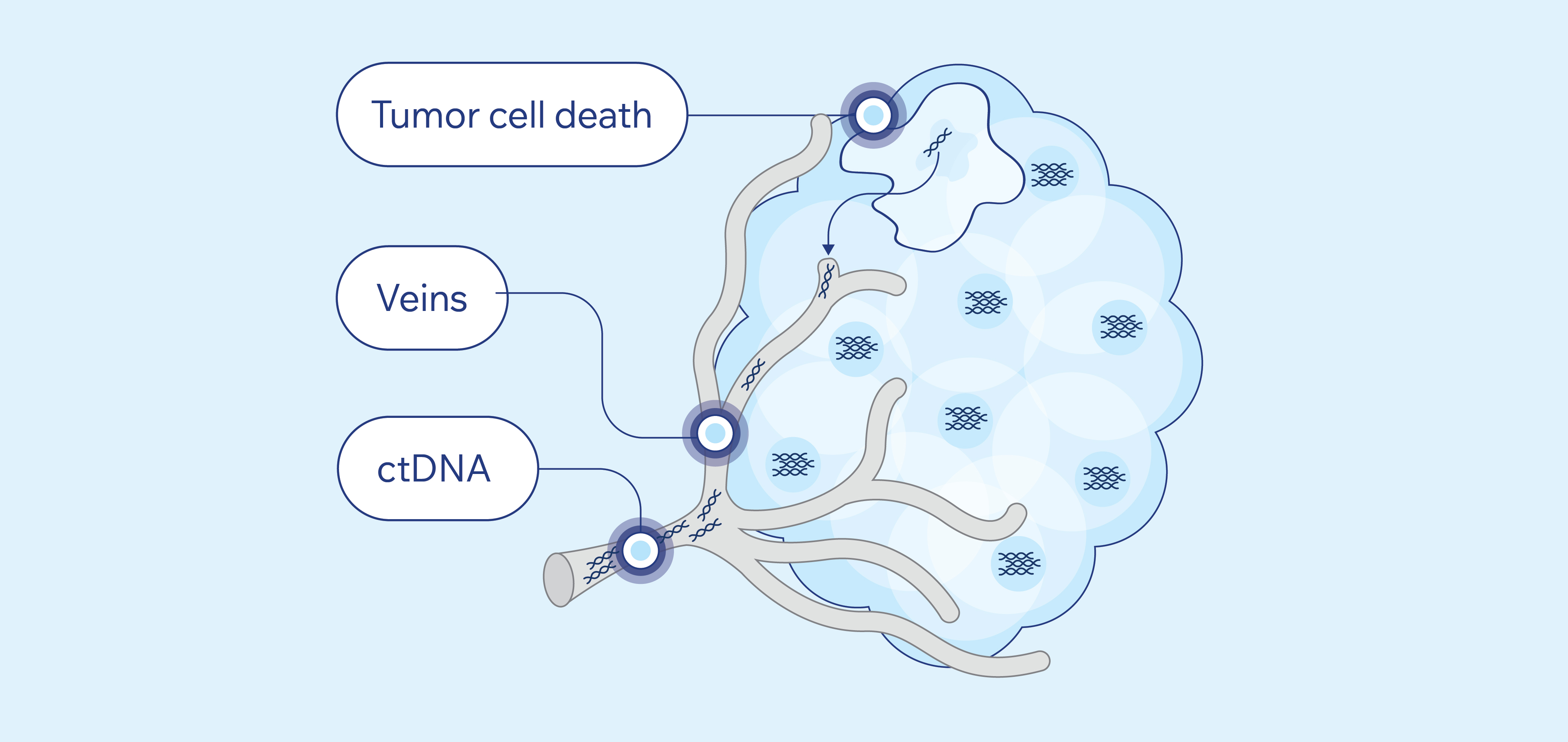
Circulating tumor DNA (ctDNA) is fragmented DNA from your tumor cells found in the blood. When tumor cells die, they release ctDNA into the bloodstream where it circulates. Blood can be tested for the presence of ctDNA or the amount of ctDNA, and the ctDNA in blood can be isolated and tested for mutations.
Testing of ctDNA can be used to
ctDNA can be both a prognostic biomarker and a predictive biomarker. ctDNA gives information about the likely outcome of the disease (prognosis) and it can be used in ctDNA guided treatment decision-making to predict treatment success and failure.
ctDNA testing after surgery for stage II or stage III colorectal cancer, testing for the presence of ctDNA in your blood can provide information about whether you have any remaining cancer cells in your body (minimal residual disease, MRD).
During follow-up care after treatment, the presence of ctDNA (ctDNA detection) can indicate a recurrence of your colorectal cancer (CRC).
In cancer patients with stage IV / metastatic colorectal cancer (mCRC), measurement of the amount of ctDNA in blood before and during treatment can predict response to chemotherapy and immunotherapy. This is called serial ctDNA measurement. A decreasing ctDNA level during treatment predicts a good response to therapy, while an increasing level of ctDNA during treatment predicts treatment failure and disease progression.
ctDNA mutation testing, also known as ctDNA molecular profiling, can be used to get information about biomarker status. It is used when there is not enough tumor tissue available for tissue-based testing, when quick results are needed, or when it is suspected that there has been a change in biomarker status in the time since a tissue sample was analyzed. Microsatellite stability (MSS) and microsatellite instability (MSI), tumor mutational burden (TMB), as well as KRAS mutations, NRAS mutations, BRAF mutations, NTRK fusion, and HER2 amplification can all be analyzed with ctDNA testing.
There are ongoing clinical trials being done at cancer centers around the world looking at how to make the most of ctDNA testing in caring for patients with colorectal cancer. Talk to your oncology team about whether you could benefit from enrollment in a clinical trial.
ctDNA is tested in a blood sample. This is called a liquid biopsy. Some tests also use a tissue biopsy sample to create a personalized ctDNA blood test or to compare ctDNA to DNA taken directly from your tumor tissue. ctDNA is analyzed using next-generation sequencing (NGS).
The results of ctDNA testing will vary depending on the type and goal of ctDNA testing.
If ctDNA detection is being used to look for minimal residual disease (MRD) after surgery, or for early detection of cancer recurrence during follow-up care, your ctDNA status may be reported as “ctDNA positive” or “ctDNA detected” or as “ctDNA negative” or “ctDNA undetectable”.
If the amount of ctDNA is measured to monitor treatment response in colorectal cancer, the numerical amount will be reported. Often, the percent that this number has decreased or increased since the pre-treatment measurement will be included.
If circulating tumor DNA analysis is being used for biomarker testing (molecular profiling), either as the main biomarker profiling method or as a study of changes in biomarker status over time, your results will be reported as they would be for those tests if they were performed on tissue. For example, ctDNA testing of KRAS would be reported as “KRAS wild-type” or “KRAS mutant”. And ctDNA testing of tumor mutational burden (TMB) would be reported as “TMB-Low” or “TMB-High”.
For more information about how ctDNA molecular profiling results are reported, please refer to the specific biomarker tested (for example, KRAS, TMB, or HER2).
Adjuvant therapy is a treatment, such as chemotherapy or targeted therapy, given after the main treatment (surgery) to treat any remaining cancer cells in the body and prevent recurrence.
There are no standardized recommendations for ctDNA testing in colorectal cancer (bowel cancer). Talk to your oncology team about whether ctDNA testing could be useful for you. ctDNA analysis may be used in testing for other biomarkers when tumor (tumour) tissue is unavailable.
A biomarker is a piece of information about your health. Biomarkers include your blood pressure, your blood type, and cholesterol or blood sugar levels measured in a blood test. The biomarkers of cancer are also known as tumor markers.